What Is Astralean and What Does It Contain (Clenbuterol HCl 40 mcg)
Astralean is a brand-name pharmaceutical product whose active ingredient is clenbuterol hydrochloride. It is most commonly encountered in the form of oral tablets labeled as containing 40 micrograms (mcg) of clenbuterol HCl per tablet. This figure refers exclusively to the amount of active substance present in each tablet and should be understood as a quantitative labeling element, not as a recommendation or guidance for use.
From a pharmacological perspective, clenbuterol belongs to the group of β₂-adrenergic receptor agonists. These substances were originally developed for their ability to relax smooth muscle tissue, particularly in the bronchial tree. Astralean, therefore, is neither a dietary supplement nor a hormone-based product. It is also not an anabolic steroid, despite frequent online confusion and its frequent mention in non-medical contexts. Chemically and pharmacodynamically, clenbuterol is a sympathomimetic agent, meaning it mimics certain actions of the sympathetic nervous system.
The distinction between Astralean and clenbuterol is important. Astralean refers specifically to a commercial formulation produced and labeled as a finished pharmaceutical product. Clenbuterol, by contrast, is the active molecule itself, which may exist in different formulations, brands, or regulatory contexts. When people speak broadly about “clenbuterol,” they may be referring to the molecule in general, to veterinary preparations, or to non-medical use patterns. Astralean represents only one branded expression of that molecule.
Astralean tablets are manufactured as a fixed-strength dosage form, meaning that each tablet contains the same microgram quantity of the active substance. Product packaging typically includes the product name, the stated strength (40 mcg), batch or lot identification, and manufacturer information. These elements serve purposes of identification, quality control, and traceability, which are standard requirements in pharmaceutical production. Since clenbuterol is active at very low doses, its measurement in micrograms rather than milligrams reflects its high pharmacological potency. Drugs with this profile tend to have a narrower margin between desired and undesired systemic effects, which partly explains why clenbuterol-containing products occupy a complex position in modern medicine.
In summary, Astralean should be understood as a branded clenbuterol tablet, designed as a pharmaceutical product rather than a wellness or performance aid. Any further interpretation of its role requires an understanding of its clinical origins, regulatory status, and mechanism of action, which are addressed in the following sections.
What Is Astralean Prescribed for in Clinical Practice
However, unlike many modern bronchodilators that are administered by inhalation and act mainly within the lungs, clenbuterol is systemically active. After oral administration, it circulates throughout the body and interacts with β₂-receptors in multiple tissues. This characteristic has important clinical implications. While systemic activity can prolong bronchodilatory effects, it also increases the likelihood of effects outside the respiratory system, particularly in the cardiovascular and nervous systems. Because of this profile, clenbuterol never became a standard first-line therapy in mainstream human respiratory medicine in many countries. In some regions, its use in humans has been restricted or discontinued in favor of more selective or locally acting alternatives. In other regulatory frameworks, clenbuterol has been more widely used in veterinary medicine, especially for respiratory conditions in animals, which has further shaped its modern perception.
Where clenbuterol is prescribed in a medical setting, it is typically done with careful consideration of patient-specific factors, including cardiovascular health, comorbid conditions, and potential drug interactions. Its use is not routine and generally reflects selective or exceptional clinical circumstances rather than standard practice.
It is also important to note that the existence of a branded product such as Astralean does not imply broad approval or routine prescription. Pharmaceutical branding often persists even when clinical use becomes limited or geographically constrained. As a result, Astralean occupies a space where its historical medical purpose is clear, but its current role in human medicine is narrow and highly regulated. This clinical background helps explain why Astralean is frequently discussed outside of conventional medical settings while remaining relatively uncommon in everyday respiratory care.
Mechanism of Action (β₂-Agonist) — Explained in Simple Terms
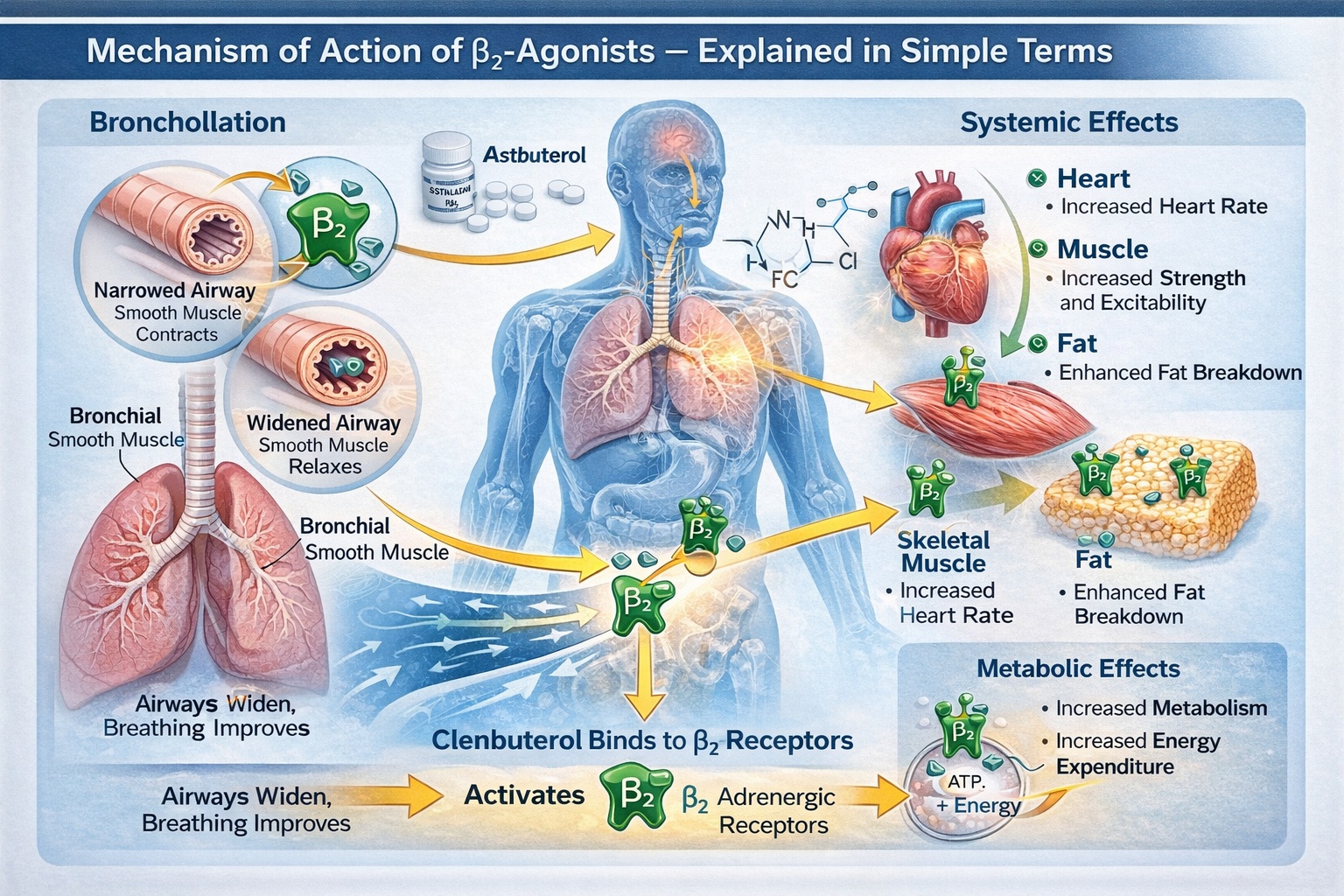
In simplified terms, the sequence is straightforward: clenbuterol activates β₂-receptors, smooth muscle tone decreases, and bronchial passages become less constricted. This mechanism underlies its original medical use as a bronchodilator.
What distinguishes clenbuterol from many commonly used respiratory drugs is that it is not limited to local action in the lungs. Because it is administered orally and absorbed systemically, it reaches β₂-receptors throughout the body. These receptors are also present in skeletal muscle, vascular tissue, and metabolic organs. Activation in these sites explains why clenbuterol can produce effects that are unrelated to breathing.
Systemic β₂-receptor stimulation may increase heart rate, enhance neuromuscular excitability, and influence energy metabolism. These effects are not incidental; they are direct extensions of the same pharmacological mechanism responsible for bronchodilation. The body does not distinguish between “desired” and “undesired” receptor activation. The same signal acts wherever the receptor is expressed. Another important characteristic of clenbuterol is its relatively long duration of action compared with short-acting inhaled β₂-agonists. Prolonged receptor stimulation can extend both therapeutic effects and systemic responses, which is one reason why clenbuterol requires greater caution than agents designed for rapid, localized relief.
From a clinical standpoint, this mechanism explains both sides of clenbuterol’s profile. On one hand, it can effectively relax airway smooth muscle. On the other, its lack of tissue selectivity increases the likelihood of cardiovascular and neurological effects, particularly in sensitive individuals or at higher systemic exposure.
Understanding this mechanism is essential for interpreting the drug’s medical limitations, safety considerations, and the reasons it attracts attention beyond its original therapeutic intent.
Forms and Labeling (40 mcg Tablets, Packaging, Identification)
Astralean is commercially available as oral tablets, with each tablet labeled as containing 40 micrograms of clenbuterol hydrochloride. This fixed-strength tablet format reflects standard pharmaceutical practice for substances that are active at very low concentrations and require precise quantitative control.
The labeling on Astralean packaging is primarily intended for product identification and traceability. It typically includes the product name, the stated strength per tablet, manufacturer information, batch or lot numbers, and an expiration date. These elements allow the product to be tracked through production and distribution channels and enable quality control measures in the event of recalls or adverse event reporting.
The presence of a microgram-level strength on the label is significant from a pharmacological standpoint. Drugs measured in micrograms rather than milligrams are generally considered highly potent, meaning that relatively small amounts are sufficient to produce systemic biological effects. For this reason, the numerical value printed on the packaging should not be interpreted intuitively or compared directly to more familiar medications with milligram dosing. It is also important to clarify what labeling does not communicate. The stated “40 mcg” refers solely to the amount of active substance contained in one tablet. It does not indicate a standard dose, a safe amount, or an appropriate frequency of use. In regulated medical practice, such decisions are determined by clinical assessment, not by packaging information alone.
From a medical and regulatory perspective, proper labeling serves as a safeguard rather than an instruction manual. It ensures that the identity and strength of the product are transparent, while leaving decisions about appropriateness and use to qualified healthcare professionals. This distinction is especially relevant for drugs like clenbuterol, where systemic effects and individual sensitivity can vary widely. Astralean’s tablet form and labeling reflect its status as a pharmaceutical product with defined potency, rather than a consumer-oriented preparation designed for unsupervised use.
Who Should Not Take It Without a Doctor’s Assessment (Red Flags)
Since clenbuterol acts on the sympathetic nervous system and produces systemic physiological effects, its use raises specific safety considerations. These concerns are not limited to high doses or prolonged exposure; they arise from the drug’s basic mechanism of action and its interaction with β₂-adrenergic receptors throughout the body.
One of the primary areas of concern is the cardiovascular system. β₂-receptor stimulation can influence heart rate, myocardial excitability, and vascular tone. In individuals with pre-existing heart disease, rhythm disturbances, or poorly controlled blood pressure, these effects may increase the risk of clinically significant complications. Even in otherwise healthy individuals, sympathetic overstimulation can provoke palpitations or discomfort that warrants medical evaluation.
Endocrine conditions also matter. In people with thyroid disorders, particularly hyperthyroidism, sympathetic activity is often already elevated. Adding a β₂-agonist into this physiological context may amplify symptoms such as nervousness, tremor, or cardiovascular strain. Similarly, individuals with anxiety or panic disorders may experience worsening of symptoms due to the drug’s stimulatory profile.
Pregnancy and breastfeeding represent another area where caution is essential. Clenbuterol crosses biological barriers and has systemic activity, making unintended fetal or neonatal exposure a theoretical and ethical concern. For this reason, any consideration of use in these populations requires specialist oversight.
Drug interactions further complicate the picture. Concomitant use of other substances that stimulate the sympathetic nervous system, including certain prescription medications, over-the-counter products, or recreational stimulants, can lead to additive or unpredictable effects. The combined impact on heart rate and blood pressure may exceed what would be expected from either substance alone. The central issue is that clenbuterol does not act in isolation on a single organ system. Its effects are integrated into broader physiological networks, which vary significantly between individuals. This variability makes unsupervised use particularly risky and underscores why medical assessment is essential before exposure.
A more detailed examination of adverse effects, contraindications, and underlying risk mechanisms is provided in Article.
Briefly: Why Is Astralean So Popular for Fitness?
Although Astralean was developed in a medical context, much of the modern attention surrounding it comes from fitness and bodybuilding communities. This shift in focus is not driven by approved therapeutic indications, but by the broader systemic effects associated with β₂-adrenergic receptor stimulation.
Clenbuterol’s influence on sympathetic activity and energy metabolism has led to the perception that it can alter body composition. Over time, these perceived effects have been amplified in online forums, anecdotal reports, and informal guides, often detached from the drug’s original pharmacological purpose. As a result, Astralean is frequently discussed less as a bronchodilator and more as a performance- or physique-related substance. It is important to emphasize that this type of interest represents off-label and non-medical use. The claims circulating in fitness-oriented spaces are rarely grounded in controlled clinical research and often minimize the complexity of clenbuterol’s systemic actions. Simplified narratives tend to focus on isolated outcomes while overlooking cardiovascular strain, neurological effects, and individual variability in response.
This gap between pharmacology and popular representation helps explain both Astralean’s visibility and the controversy surrounding it. A drug designed to act on fundamental physiological signaling pathways does not lend itself well to casual or experimental use, yet its reputation has evolved in precisely that direction.
A critical analysis of fitness-related claims, along with an examination of associated risks and misconceptions, is addressed in Astralean and Weight Loss / Sports: What the Evidence Says, Why It’s Banned, and What the Dangers Are.
FAQ
What is Astralean?
Astralean is a branded pharmaceutical product containing clenbuterol hydrochloride, a β₂-adrenergic agonist originally developed for its bronchodilatory effects. It is produced as a fixed-strength tablet rather than a variable or compounded preparation.
Is Astralean the same as clenbuterol?
Astralean is not a separate substance. It is a brand name, while clenbuterol is the active molecule. Different brands may contain the same active ingredient, but they can differ in labeling, manufacturing standards, and regulatory context.
Astralean tablets 40 mcg: what does the number on the package mean?
The “40 mcg” designation indicates the amount of clenbuterol hydrochloride contained in a single tablet. It reflects chemical content only and should not be interpreted as guidance on how much is appropriate or safe for an individual.
Is Astralean legal?
The legal status of clenbuterol-containing products varies by country and by context, such as human medicine, veterinary use, or non-medical possession. Regulatory distinctions and legal frameworks are discussed in Article 4.
Is Astralean considered a steroid or hormone-based drug?
No. Astralean does not contain steroids and does not act through hormonal pathways in the way anabolic–androgenic steroids do. Clenbuterol is a sympathomimetic β₂-adrenergic agonist, meaning its effects are mediated through nervous system signaling rather than endocrine hormone receptors.
References
- Alpha Pharma Healthcare. (n.d.). Products. https://www.alpha-pharma.com/products.php
- DrugBank Online. (n.d.). Clenbuterol. https://go.drugbank.com/drugs/DB01407
- WebMD. (n.d.). Clenbuterol: What you need to know. WebMD. https://www.webmd.com/drugs/what-you-need-to-know-about-clenbuterol-for-bodybuilding
- ScienceDirect Topics. (n.d.). Clenbuterol. Elsevier. https://www.sciencedirect.com/topics/medicine-and-dentistry/clenbuterol





